While assessing the safety and efficacy of medicines is the bedrock in drug research, vaccine development during the COVID-19 pandemic highlighted the changes in the types of medicines developed today and the stakeholder management required for successful adoption. Enabled by new treatment paradigms, digital transformation, advanced technology applications, data availability, and the patient-centric focus, the changing ecosystem requires new capabilities in assessing the safety of medicines and managing the new entities at play. This Viewpoint explores strategies for innovative companies to excel with the industry shift, focusing on pharmacovigilance (PV) as a strategic advantage.
PHARMACOVIGILANCE HAS NEVER BEEN MORE CRITICAL IN DRUG DEVELOPMENT
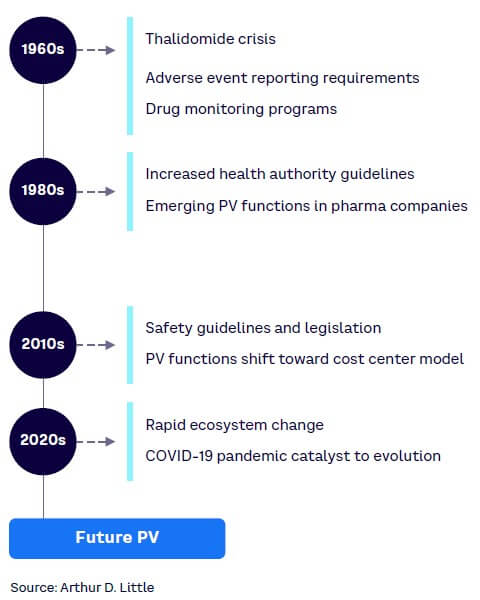
The drive for safe and effective medicines has accelerated since the thalidomide tragedy in the early 1960s and has led to the implementation of a variety of disciplines to ensure the public is protected (see Figure 1). From a health authority (HA) perspective, many regulations have been introduced, with dedicated agencies overseeing drug approvals, licensing, and use. For drug developers, structures, standards, and processes have been put in place to ensure they comply with the applicable regulations and ethical values. The use of medicines and vaccines must protect patients and safeguard public health.
However, the controversial discussion surrounding the safety of the COVID-19 vaccines moved PV (the science and activities related to adverse effects of medicines and vaccines) into the general consciousness to an extent never before seen. This discussion also put PV in the middle of a heated, often political, debate and contributed to the negative sentiment toward some COVID-19 vaccines and the refusal by a portion of society to take those vaccines.
What is clear in today’s drug development process is that the positive treatment effects — the “efficacy” — tend to prevail in the discussion, while drug “safety” may not always be seen as a strategic input but more of an obligation. The regulation-mandated obligations to report adverse events (AEs) and adverse drug reactions (ADRs) mean PV is often regarded as a “data collecting and submitting” office — a post-box-like team. Aligned with this perceived view, PV departments have developed operational excellence regarding case processing and reporting (expedited and aggregated alike). They frequently employ external service providers as well as smart IT solutions aimed at touchless case handling and cost efficiency. This operational focus is frequently reflected in and maintained by organizational structures, capabilities, and resources, as well as the emphasis on efficacy during drug development.
PV AUTOMATION ALONE WILL NOT SUSTAIN ECOSYSTEM CHANGES
The global COVID-19 pandemic identified the need for a different approach to science and healthcare — one that includes an unmistakable call for the appropriate use of PV scientific excellence that considers the expected multifold changes that are occurring in the types of medicines being developed and/or the way they are developed and approved. These transformations, be it in drug modalities (e.g., mRNA-based compounds), digitalization, innovative clinical trial design, or complexities in the regulatory pathways, all point to the fact that PV’s operational excellence and focus on automation of case processing will not be sufficient in the future.
The call to review PV’s value proposition at this turning point is of special importance for established companies that are undergoing major transformations and for the rising stars that jump from pure research to marketing drugs in almost no time.
DISRUPTORS HERE TO STAY
While COVID-19 vaccine development raised legitimate questions, we believe that it is emblematic of the disruptions that are driving the ecosystem change. As with any seismic shift in an industry, businesses that are aware, ready, and equipped for the changes will be best positioned to thrive. For PV, organizations will have to address the following three areas.
1. New treatment paradigms
Precision medicine (also referred to as personalized treatment or targeted therapies) contrasts significantly with the traditional one-approach-fits-all model. It aims at improving health outcomes by utilizing genomic, proteomic, metabolomic, and other biomarkers. Genotyping is described in some labels as a predictive safety measure (e.g., carbamazepine and abacavir), but pharmacogenomics is not broadly introduced in the PV routine. There are only a limited number of randomized clinical trials that demonstrate the advantages of drug selection and dose adoption for the individual patient in reaching the drug’s optimal benefit-risk ratio. For various reasons, the few trials that exist do not prove the predictability of the biomarker for recommending genotype-guided treatment.
Over the past three decades, biologics have revolutionized drug development. These mostly high-cost compounds offer new therapeutic options for several chronic and sometimes life-threatening diseases (e.g., in oncology, immunology, and various rare diseases). The COVID-19 pandemic accelerated the use of transformative medicines and especially mRNA technology. While addressing unmet patient needs is exciting and rewarding, the novel treatments, such as chimeric antigen receptor (CAR)-T cell and curative gene therapies, require a transformation of PV expertise compared to the established, chemical-synthesized small molecule drugs.
2. Digitalization & access to more & new data
Electronic health records, the Internet of Things, and medical devices linked to the cloud enable the generation of an unprecedented and astronomically high amount of data. Devices, such as wearables and apps, are increasingly used to allow participation in clinical trials, monitor patient progress, and collect and handle data. Digital technology can provide access to routine and additional risk-mitigation activities in clinical practice (e.g., package inserts). However, various areas require clarification, including the following questions:
-
What are the consequences for PV regarding the information coming from, for example, an activity tracker?
-
Is there an obligation for a pharmaceutical company to collect, process, and report “accidental information”? And, if so, what would be the added value for a product safety assessment using that data?
-
Is there an adverse event reporter in data collected from social media?
Digital symptom management using smart tools may improve patient safety, quality of life, and outcome. Moreover, these systems could have the functionality to capture and analyze real-world data. Various workstreams at the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), and the US National Institutes of Health (NIH) are also working with experts from epidemiology, statistics, and computer science, among others, on strategies for the effective management and analyses of these data streams. At present, there is a lack of clarity regarding the usage and acceptance of real-world data and patient-reported outcomes within regulations.
With the digital and technological advances alongside the potential regulatory grey zones, PV organizations must now prioritize their strategy for handling and using the inbound digitally enabled data, both from trials and post-marketing.
3. The shift to “real” patient-centricity
It may sound provocative, but PV departments have tended to consider HAs as their key partners rather than the patients. Understandably, this has been due to the strong focus on compliance and the perception of HAs as the exclusive advocates of patients. However, there is now a shift to putting the “real” patient first to achieve the best experience and outcome for them. This poses challenges for PV organizations, such as:
-
Thus far there is no established definition of patient-centricity or a general standard framework for patient-centric approaches. The heterogenicity of terms hinders the identification of PV’s role in discussions with research and development, commercial, legal, regulatory affairs, and so on, on the essential role PV can play for patient-centered-outcome delivery.
-
Proactive, fact-oriented, and consistent communication with understandable messages regarding product safety and risks plays a key role in gaining patients’ confidence in drug treatment and maintaining treatment compliance. Yet PV is not generally at the forefront of these communications. The COVID-19 pandemic revealed many examples of the impact of miscommunications on vaccine safety.
Various companies have recently added “patient safety” to their PV department name to reflect the commitment to patient involvement in medical decision making, their preferences, and perspectives. This is a first step and a move in the right direction.
REINVENTING PHARMACOVIGILANCE
In response to the changing ecosystem of drug development and new treatment paradigms, we believe the way forward for drug developers is a shift to a forward-looking and empowered PV operating model. This requires various building blocks, as outlined below.
Early PV involvement with new and/or digital-enabled projects
The importance of patient safety mandates a leading role for PV science to enable the smart use of experts and personnel and in preventing data disasters. This definitively includes activities conducted under the umbrella of market research programs and patient support programs that have increasingly gone digital as well as the use of other real-world data.
The shift in gear required for the future of health is one where PV teams are at the forefront of conducting new technology and data type assessment instead of the current reactive model. PV units will have to inject new capabilities — from machine learning specialists to health technology assessment experts — to be able to guide the business on new digital-enabled strategies and projects.
Furthermore, PV units will need to develop or contribute to a framework and training to ensure that the way a company is using advanced tools and digitalization adds value to PV topics and is compliant with respective regulations.
Accelerating “real” patient-centricity
As stated earlier, effective communication regarding product safety and risks plays a key role in enabling patients’ confidence in new drug therapies and in maintaining treatment compliance. Effective communication requires PV leadership and PV resources, proactive planning, and intense collaboration with internal and external partners, as well as the willingness and skills for mutual understanding.
Future PV will require dedicated sub-functions with the sole responsibility of direct engagement with patients and patient groups through their preferred communication channels to collate questions and concerns and to more effectively translate safety-related information to the layperson. This will involve new ways of working internally as well as increased engagement with functions like patient advocacy, medical affairs, medical communications, and commercial to ensure a holistic view of the end customer.
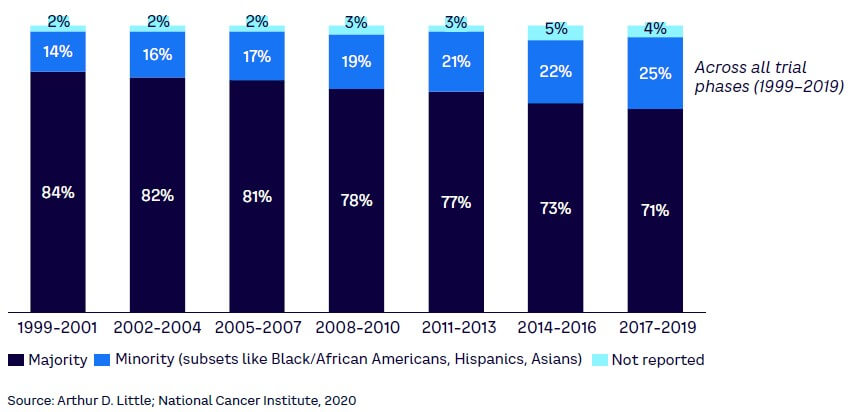
Furthermore, the safety profiling of medicinal products and the evaluation of the benefit-risk balance historically have been conducted with information that does not represent a diverse population. Underrepresented groups lead to knowledge gaps regarding safety between trials and real-world scenarios with an impact on confidence in medicinal products and latent unforeseen consequences for the impacted patient group (see Figure 2). PV will have to play a significant role in closing the gaps in trial diversity.
New talent acquisition
Novel therapies, advanced therapy medicinal products (ATMPs), and new methods in drug development require new talent in PV with the qualifications and expertise needed to define the risk profile of the newer, highly individualized treatments, such as CAR-T cell therapy. Knowledge in pharmacogenomics and clinical immuno-safety, aiming for high-quality standards, ensuring reliable information for advances in signal detection, and identifying the frequency of ADRs in overall low patient numbers can be challenging. Multi-stakeholder dialogue is a crucial tool in guiding these new therapies, and PV should assume accountability for navigating the less worn paths. Companies will have to acquire talent with the right expertise or invest in developing these internally.
External partnerships
Challenges and opportunities regarding innovative and/or complex trial designs, accelerated patient recruitment, regulatory fast-track designations, public-private partnerships, and so on, can only be addressed by multi-stakeholder partnering. Relevant partners should come together from the pharmaceutical industry, academia, HAs, and patient organizations. These new models work best when roles and responsibilities are clearly detailed and mutually agreed upon, such as in a PV agreement, and when PV has the opportunity to contribute to public consultations.
Conclusion
ENSURING SUCCESS WITH PV
Giving patients access to game-changing, high-quality, and safe treatments within the shortest possible time frame is a pharmaceutical industry mandate. PV has a crucial role in this environment of accelerated transformations. PV departments and associates should be tasked to demonstrate the added value that they bring to drug development and marketed products, beyond pure data handling and operational proficiency:
-
Give PV a seat on the leadership team with a clear mandate to provide input into the strategy of new treatment paradigms, accelerated trial designs, and new marketing concepts before commencing clinical trials.
-
Set up a global PV organization with strong local input and constant interdepartmental exchange, anchored high in a company’s hierarchy.
-
Establish measures to attract, build, and retain new PV talent. Also, assign appropriate resources with a clear mandate to build the required capability for the new ecosystem.
-
Drive a culture where all employees, from top to bottom, share a compliant PV system and PV compliance is a company-wide task that does not stop at the PV department door.
-
Involve PV very early in the company’s digital projects, not to add bureaucracy but to make the best use of the activities and to guarantee compliance.
-
Give PV science a leading role in initiatives and workstreams, looking at how to generate additional, meaningful, and compliant data from existing information.
-
Train PV experts to efficiently communicate the facts regarding product safety and contribute to answering questions on the benefit-risk of medicinal products and vaccines.
-
Create networks with other drug developers, academia, and HAs to benefit from concerted actions and learn from each other.
Increasing reliance on PV expertise for patient and product safety topics throughout clinical development, at filing, at product launch, and throughout the lifecycle of a medicine gives PV higher visibility and an opportunity to add value. PV must leave the post-box silo and the pure focus on operational excellence and instead demonstrate accountability and show it is prepared to address the needs of the different treatment paradigms, the changing healthcare system, and the evolving science landscape. A strong and well-anchored PV department gives a company the flexibility to adapt to challenges and take on future projects.




